
Lipella Pharmaceuticals is a clinical-stage biotechnology company developing and commercializing treatments for serious diseases. Our proprietary drug delivery technology has potential applications in addressing diseases of the mucosal tissue including the bladder, urethra, oral cavity, esophagus and colon. We see strong potential for our technology in partnerships and commercial licensing agreements.
Lipella maintains a sterile manufacturing facility in Pittsburgh, PA for the production of clinical supplies and research products.
Click the button above to view Lipella Corporate Presentation
February 2025

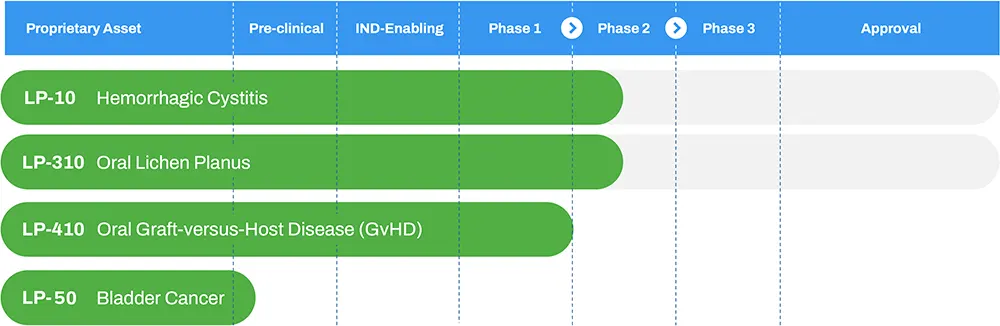
We have completed a Phase 2A multi-center dose-escalation clinical trial for our lead product, LP-10, with results published in the peer-reviewed journal International Urology and Nephrology. Our LP-310 program for Oral Lichen Planus has shown positive topline results in its Phase 2a trial. Additionally, we received Orphan Drug Designation for LP-410 in the treatment of oral Graft-versus-Host Disease (GVHD).
We maintain a pipeline of additional product candidates aligned with our strategy of developing proprietary 505(b)(2) assets targeting highly-morbid conditions with no adequate treatments.

Reach Out