Lipella Pharmaceuticals Pipeline
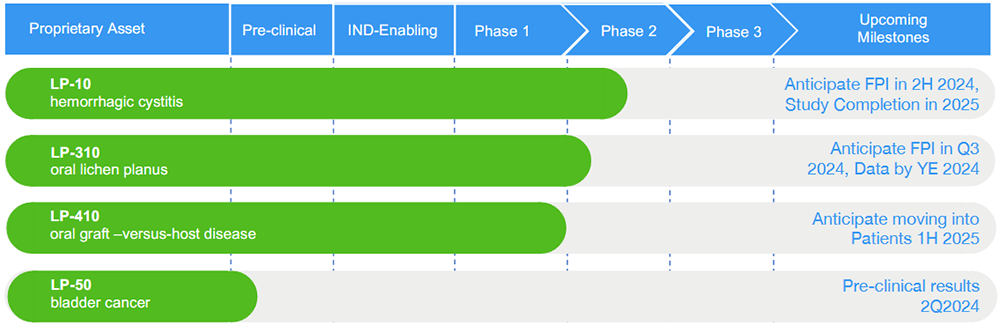
- LP-10 is an intravesical formulation intended for the treatment of moderate to severe hemorrhagic cystitis (HC), a serious disease with no adequate existing therapy. LP-10 has been granted Orphan Disease Designation (ODD) by the US FDA (link), and has generated positive top line results demonstrating preliminary safety and efficacy in a multicenter Phase 2a dose escalation trial (link); topline results published in the Journal of Urology (link), and full results published in the International Urology and Nephrology (link). The US FDA has recently granted a Type C meeting request to discuss the company’s proposed Phase-2b trial design (link). Early LP-10 research has been funded by the US National Institutes of Health (NIH) (link)
- LP-310 is an oral rinse formulation intended for the treatment of oral lichen planus (OLP), a chronic, painful, inflammatory, pre-malignant, T-cell-mediated, autoimmune disease affecting the oral mucosa of approximately 6-7 million Americans. There is no approved pharmacotherapy and most currently available treatments are palliative. Lipella has received IND approval for a Phase 2a multicenter dose escalation trial evaluating the safety and efficacy of LP-10 in subjects with symptomatic OLP (link). Lipella expects to commence dosing the first patient in the summer of 2024.
- LP-410 is an oral rinse formulation intended for the treatment of oral Graft-versus-Host Disease (GvHD), a rare and serious disease. GvHD is a major cause of morbidity and mortality with chronic GvHD being the leading cause of nonmalignant fatality post hematopoietic cell transplantation (HCT). Lipella has been granted FDA Orphan Drug Designation for LP-410 in the treatment of GvHD (link) and received IND approval for its Phase 2a clinical trial in March 2024 (link).
- LP-50 is an intravesical formulation for local, intravesical PD-1 (i.e. checkpoint) inhibition, intended for the treatment of non muscle invasive bladder cancer (NMIBC). Intravesical immunotherapy presents a promising avenue for bladder cancer treatment, offering the potential for increasing efficacy while minimizing systemic toxicity. Lipella’s abstract highlighting preclinical data regarding LP-50 was published by the American Society of Clinical Oncology (ASCO) as part of its 2024 annual meeting.

Reach Out