LP-10 for Hemorrhagic Cystitis (HC)
Lipella has successfully completed a Phase 2a, dose-escalation clinical trial evaluating LP-10 (liposomal tacrolimus) for the treatment of hemorrhagic cystitis (HC). Results were published in the peer-reviewed journal, International Urology and Nephrology. Lipella has received FDA Orphan Designation for this product. The company expects to initiate its Phase 2b trial in LP-10 in H1 2024.
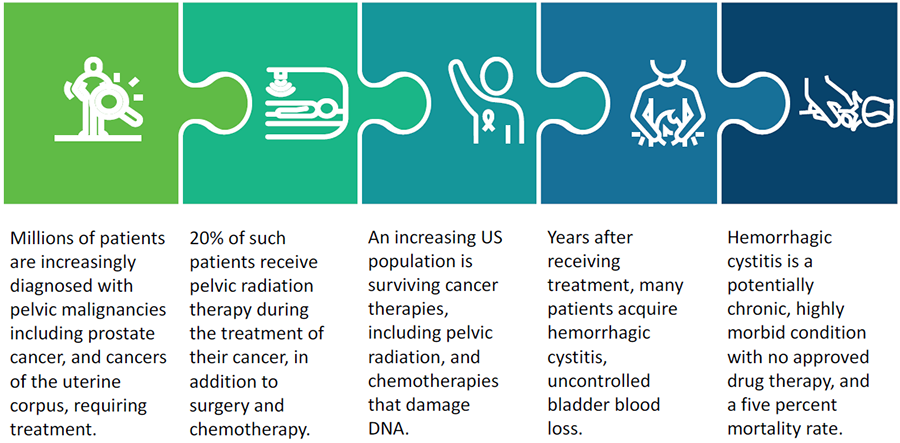
HC is serious, life-threatening bladder damage from pelvic radiation therapy and/or bladder-toxic chemotherapy. It causes chronic, painful urinary inflammation and blood loss called hemorrhagic cystitis. The blood loss, associated with hemorrhagic cystitis and radiation cystitis can lead to surgery, and can be fatal. There is currently no FDA approved drug treatment for HC.
For more information on the hemorrhagic cystitis and radiation cystitis clinical trial, please call 412-894-1853 or email [email protected].
Hemorrhagic Cystitis, Uncontrolled Urinary Blood Loss
Hemorrhagic cystitis is a serious, life-threatening bladder damage from pelvic radiation therapy and/or bladder-toxic chemotherapy.

Reach Out